It is a complex matter. Both when we are making sure that new medicines get out to patients at public hospitals; and when medicines at hospital pharmacies and on hospital shelves are to be replaced because other medicines have beaten the competition in a national tendering procedure.
Therefore, in Denmark we have established a specially structured set-up with collaboration between hospital pharmacies, pharmaceutical committees, clinical pharmacologists, economists and Amgros. We call it the Implementation Group.
Our role at Amgros is to contribute knowledge, advice and cooperation. Amgros also performs the specialist secretariat function.
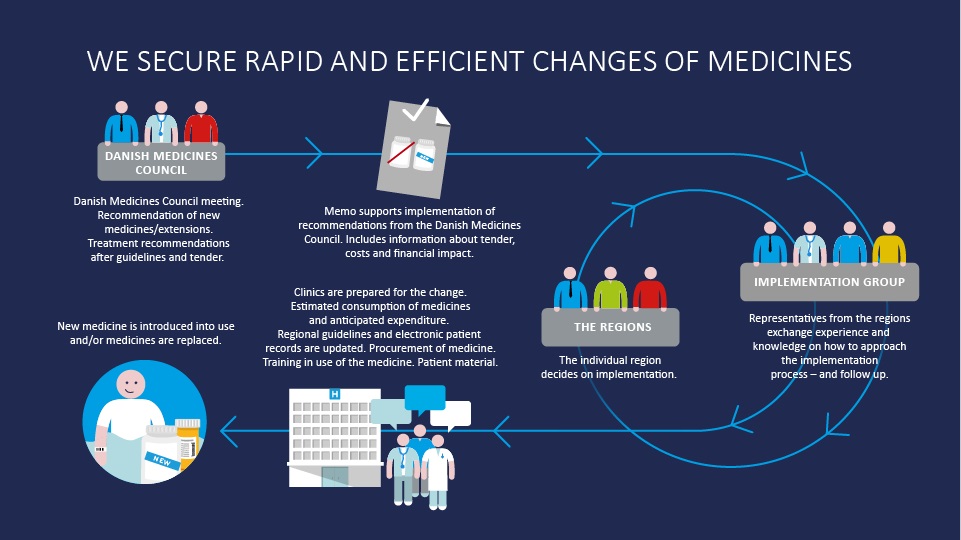
Before a completely new medicine can be brought into use as a standard treatment at Danish public hospitals, the Danish Medicines Council has to assess and recommend it. This also applies if a medicine with extended indication is to be recommended as the standard treatment.
When several medicines exist to treat a disease, Amgros will organise a tendering procedure, in which the different medicines compete in a national process. The purpose is to generate competition and achieve lower prices.
However, the expert committees at the Danish Medicines Council have to issue new treatment guidelines for a specific therapeutic area, like the pulmonary area, before Amgros can organise a tendering procedure. The treatment guidelines describe which medicines are clinically equal, i.e. equally as good to treat patients within the relevant therapeutic area. This applies both for the efficacy and possible side-effects of the medicine.
After we have found the winners of the tendering procedure, the Danish Medicines Council prepares a medicine recommendation. In the medicine recommendation, the Danish Medicines Council lists the equivalent medicines according to which have won the tender, so that hospitals can see which medicines they should choose in relation to price.
In order to help the regions implement medicine recommendations from the Danish Medicines Council, and council recommendations for new medicines and indications, Amgros draws up what we call an implementation memo. The memo contains information about the economic effects of taking a new medicine into use or of complying with a medicine recommendation. Furthermore, the memo describes various market conditions and plans for tendering procedures. In this way, we ensure that the regions can include the medicine costs in their own budgets, and that they are prepared for what will happen within a therapeutic area.
After we have prepared and issued the implementation memo, the Implementation Group will meet. The group comprises representatives from the regions - hospital pharmacies, pharmaceutical committees and clinical pharmacologists - and Amgros. All members of the group are directly involved in practical implementation. The group discusses how implementation can be carried out in practice, and the practicalities of the implementation itself. For example, if it entails a new medicine that resembles one that hospitals already use, the regions will discuss what to do. They will also talk about how fast they think they will change the medicines, how they want to do so, what resources a change will require, and whether the new medicine will require more space and different handling. They will also discuss whether patients may have to be admitted to hospital more often.
The individual regions will then each decide how they will implement the change of medicine. This is usually in a process in which the Implementation Group continuously compares consumption across the regions to learn and consult with each other, and to help establish a consensus across the regions.
When hospitals are to change a medicine, they have to update their guidelines. If a guideline, for example on rheumatoid arthritis, states that one specific medicine is to be used in a particular situation, but now another is to be used, the region will have to update its guidelines.
The same applies for information in electronic patient records. These also have to be updated to include the new medicine. The medicine also has to be replaced physically at the hospital pharmacy and in the medicine room. Furthermore, staff have to be taught how to deal with a new medicine, and patients may have to be trained in practical use.

We get new medicines into use very quickly
It only takes between one and 14 days from when the Danish Medicines Council recommends a new medicine to when the regions can order it and start using it to treat patients.
This is the result of a new analysis from the Implementation Group under the Interregional Forum for the Coordination of Medicine A structured national set-up makes this rapid process possible
READ MORE
Pharmaceuticals
Danes are living longer. And we can treat more diseases, because new and better drugs are entering the market all the time. This is good. But it’s expensive. Therefore, it is important that we are at the forefront of market developments.
READ MOREPrice negotiations and tendering
Amgros manages procurement of almost all the medicine used in Danish public hospitals. It is our task to organise tendering procedures for pharmaceuticals so that we cover hospital needs
READ MOREAnalogue competition
When the Danish Medicines Council is to assess several pharmaceuticals within the same therapeutic area, it prepares treatment guidelines.
READ MOREGeneric pharmaceuticals
When the patent on a pharmaceutical expires, and generic or biosimilar alternatives enter the market, Amgros’ task is to ensure the widest possible competition.
READ MOREContact
Jette Østergaard Rathe
Senior Medical Advisor
I work on improving and facilitating work to implement changes in medicines in the regions, so they generate value for our cooperation partners.
Mikala Vasehus Holck
Senior Medical Advisor
I work on improving and facilitating work to implement changes in medicines in the regions so they generate value for our cooperation partners.