There's great interest in alternative agreements. For this reason, Amgros works closely with the Danish Medicines Council and suppliers to strengthen and develop the area - and ensure patients have access to new, expensive and innovative medicines.
At Amgros, we systematically collect data and knowledge to support dialogue and decisions.
In the period 2021-2024, Amgros concluded 10 out of a total of 37 requests for alternative agreements received by Amgros and the Danish Medicines Council from suppliers.
There have been good reasons for some requests from suppliers not resulting in Amgros entering into an agreement.
Be aware of the discrepancy between "year of request" and "year of agreement entered into". This means that the agreements entered in 2023 do not necessarily relate to a request received in 2023. For example, the four agreements entered in 2023 can both be based on requests received in 2022 and in 2023.
Concluding alternative agreements is no quick fix. Our experience shows this.
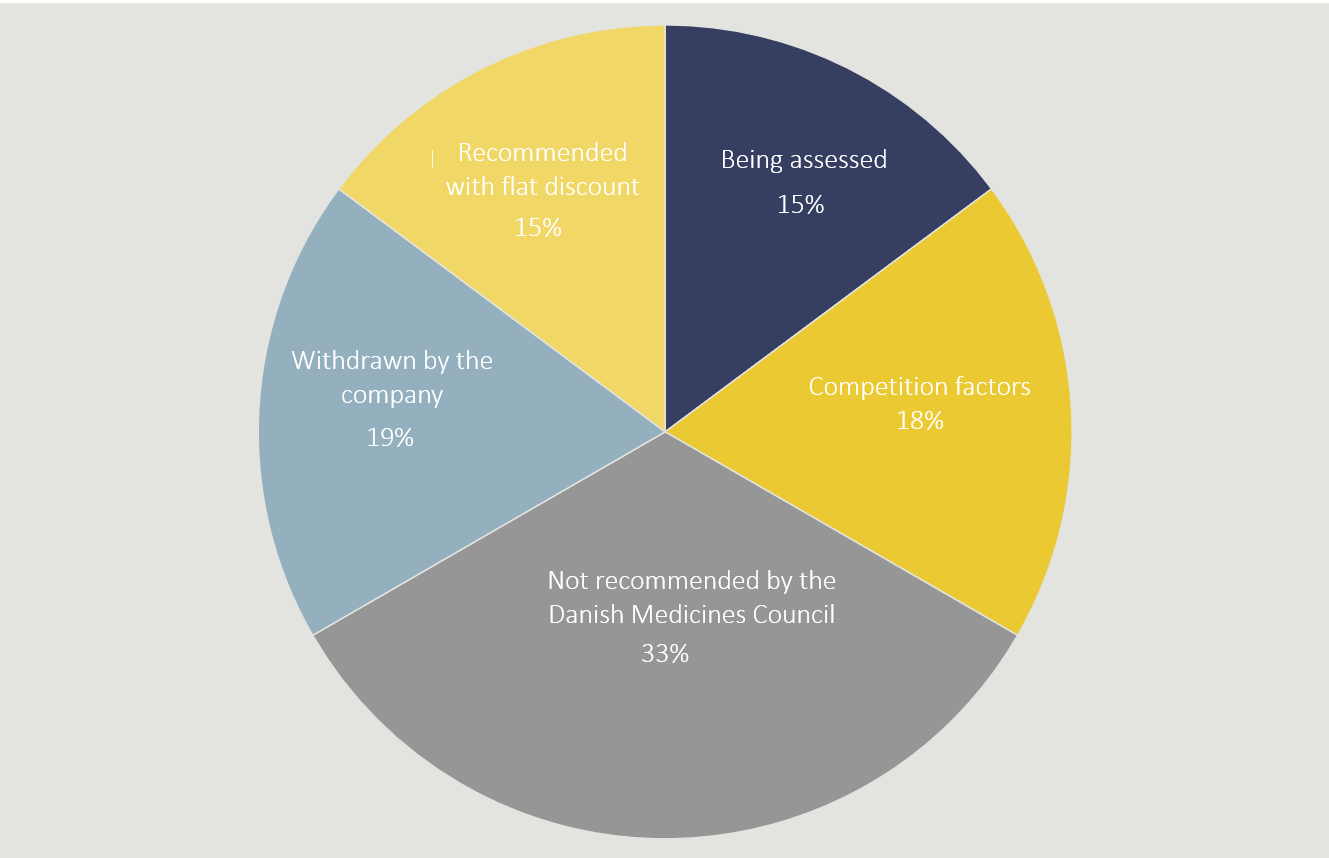
When a supplier requests an alternative agreement from Amgros and the Danish Medicines Council, the supplier also has to offer a flat discount agreement so that the Danish Medicines Council can compare the two proposed agreements. In 15 percent of the cases in which an alternative agreement is not concluded, it is because the Danish Medicines Council has preferred a flat discount agreement.
In 19 percent of the cases, the supplier chose to withdraw its request. For example, this may be because the supplier decides that the medicine is not to be introduced in Denmark anyway.
In 33 percent of cases, the medicine was not recommended for standard use by the Danish Medicines Council.
If a medicine has been, or is expected to be included in an upcoming tendering procedure, tendering regulations will often preclude Amgros from entering into an alternative agreement. This is the reason behind 18 percent of rejections.
Since 2021, Amgros has systematically collected data on the requests we receive for alternative agreement. From this data, we can see that about one-third of the requests are for an effect-based model.
The remaining requests include economic models such as a patient-initiation model, budget-cap model or price-volume model.
Read more about the different models for alternative agreements (in Danish) here.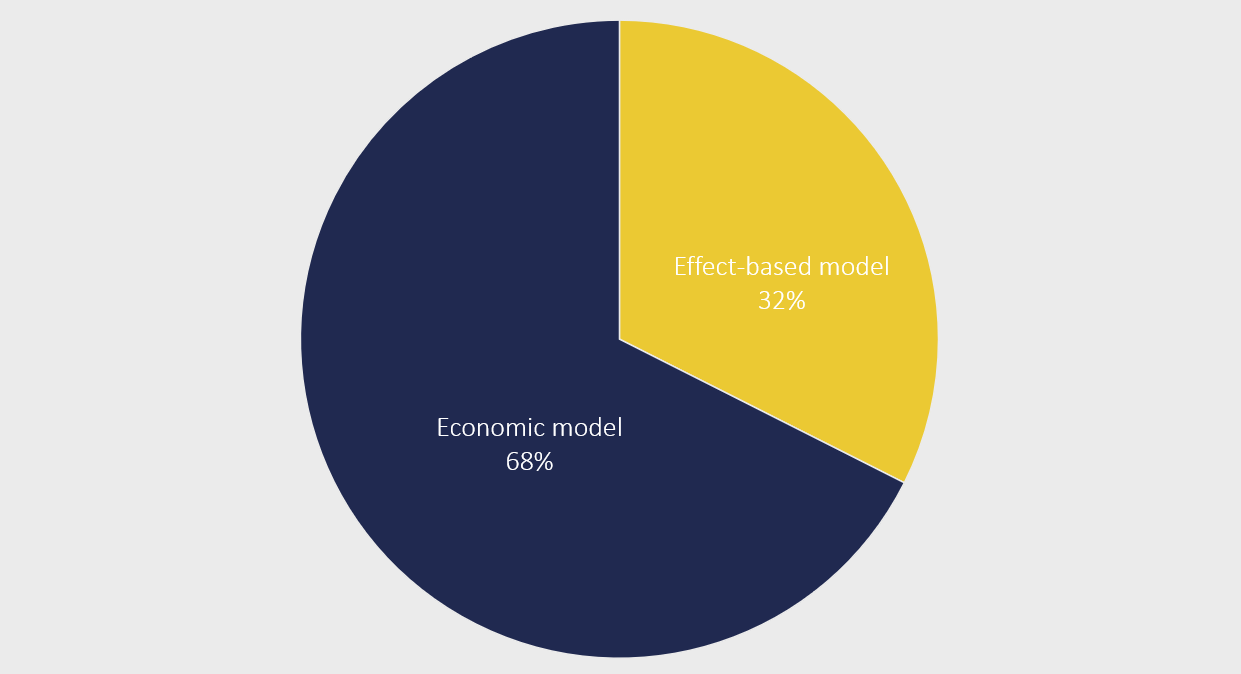
The requests for alternative agreements received by Amgros and the Danish Medicines Council relate to different disease areas.
Half of the requests are regarding solid tumours or haematological cancer.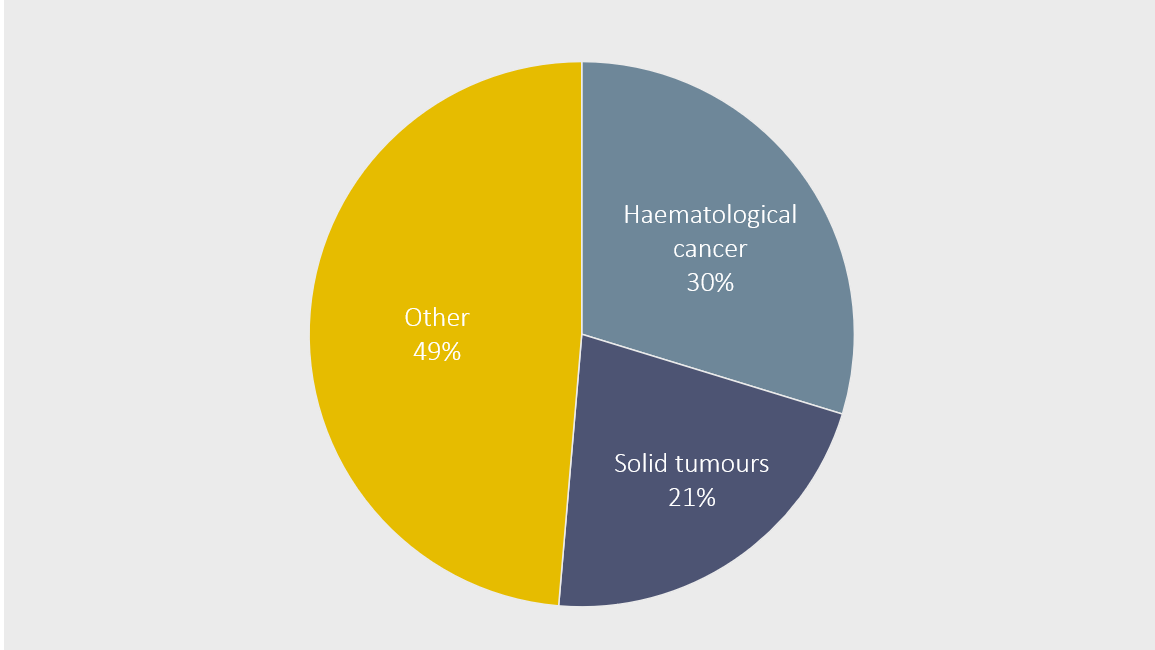
Other disease areas include: blood diseases, dyslipidaemia, endocrinology, gastroenterology, infectious diseases, immuno-defects and transplants, liver diseases, neurological diseases, neuromuscular diseases and rare hereditary diseases.

READ MORE
Price negotiations and tendering
Amgros manages procurement of almost all the medicine used in Danish public hospitals. It is our task to organise tendering procedures for pharmaceuticals so that we cover hospital needs
READ MORENew pharmaceuticals and negotiations
If a new medicine is to be considered as a standard treatment at Danish public hospitals, the Danish Medicines Council has to assess and recommend it first.
READ MOREAlternative agreements
Amgros has been using alternative agreement models for several years. These ensure that patients have access to new, expensive and innovative medicines. We apply a number of principles for using alternative agreement models. Among other things, these principles ensure that all suppliers are treated equally.
READ MORE
